Research
Flow visualization
Despite recent advances in flow diagnostic techniques, flow visualization remains a central tool in the experimentalist’s arsenal. Flow visualization allows researchers to easily highlight flow features that are cumbersome to extract from quantitative flow measurements and informs the placement of quantitative flow probes.
As part of our work, my collaborators have developed and characterized several updated versions of classical flow visualization techniques. In particular, we have characterized the properties of ammonium thiocyanate solutions for use as index-matching fluids with viscosity and density similar to that of water. While the word "thiocyanate" might bring to mind "cyanide", ammonium thiocyanate is no more dangerous or trickier to work with than other salts commonly used to make index-matching fluids such as sodium iodide.
One interesting thing that we discovered after we published our work on ammonium thiocyanate solutions is that reaction that turns the solution red as the thiocyanate ions react with dissolved iron in the water can be reversed. As Sommeria, Meyers, and Swinney point out in their book chapter in Nonlinear Topics in Ocean Physics (North-Holland, Amsterdam, 1991), the reaction can be reversed by adding small amounts of ascorbic acid to the working fluid.
More recently, we have investigated the properties of a new rheoscopic flow visualization technique based on stearic acid crystals extracted from shaving cream. Because the density of stearic acid crystals is very close to that of water, it takes them a very long time to settle out of suspension and give a rheoscopic fluid that is stable for months!
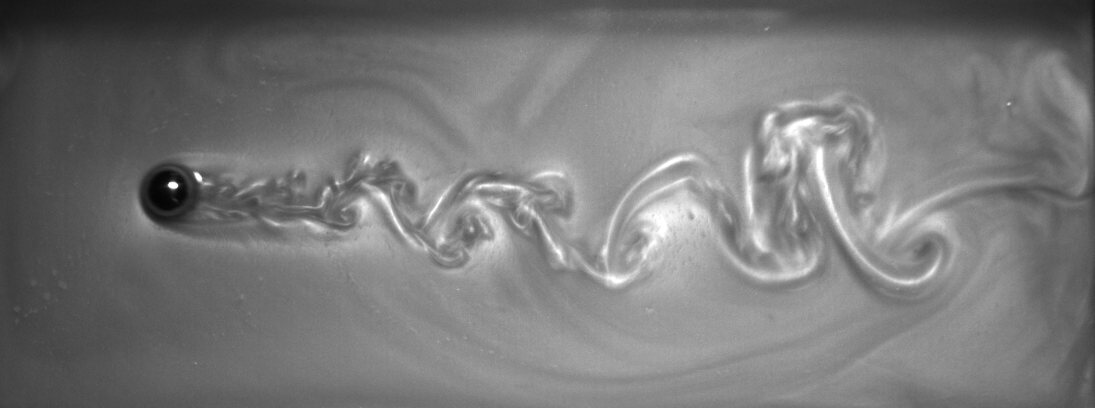
The wave behind a rolling ball bearing visualized using
rheoscopic fluid extracted from shaving cream.
Related Publications
- D. Borrero-Echeverry, C.J. Crowley, and T.P. Riddick, “Rheoscopic fluids in a post-Kalliroscope world,” Phys. Fluids 30, 087103 (2018).
- D. Borrero-Echeverry and B.C.A. Morrison, “Aqueous ammonium thiocyanate solutions as refractive index-matching fluids with low density and viscosity,” Exp. Fluids 57, 123 (2016).
© 2018 Daniel Borrero Echeverry | Last updated: 6-15-2018
